Are you ready to discover 'magnesium oxide chemistry lab report'? Here you can find questions and answers on this topic.
Mg Oxide: Percent Fruit Lab Report This is a science lab report which examines the oxidation of Magnesium and its percent yield. IT is a selfsame common lab account assignment for graduate school students crosswise North America and the UK.
Table of contents
- Magnesium oxide chemistry lab report in 2021
- Empirical formula experiment lab report
- Magnesium oxide lab answer key
- Mass data for magnesium oxide combustion reaction
- Mole ratio of magnesium to oxygen
- Magnesium oxide experiment errors
- Empirical formula magnesium oxide experiment
- Empirical formula magnesium oxide lab
Magnesium oxide chemistry lab report in 2021
 This image demonstrates magnesium oxide chemistry lab report.
This image demonstrates magnesium oxide chemistry lab report.
Empirical formula experiment lab report
 This picture illustrates Empirical formula experiment lab report.
This picture illustrates Empirical formula experiment lab report.
Magnesium oxide lab answer key
 This picture illustrates Magnesium oxide lab answer key.
This picture illustrates Magnesium oxide lab answer key.
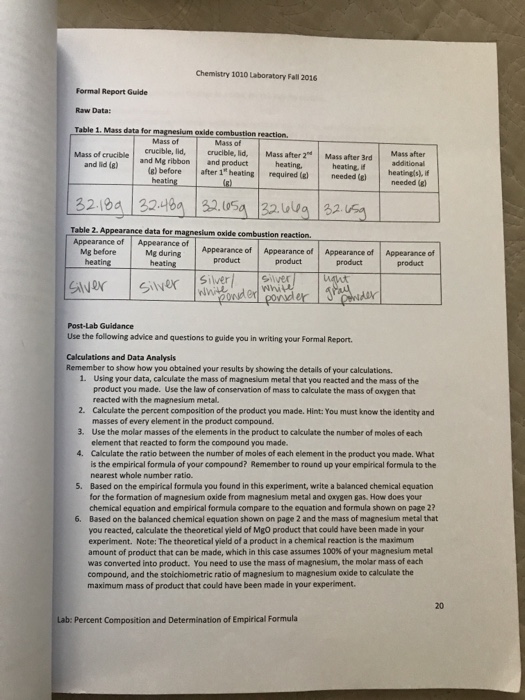
Mass data for magnesium oxide combustion reaction
 This picture illustrates Mass data for magnesium oxide combustion reaction.
This picture illustrates Mass data for magnesium oxide combustion reaction.
Mole ratio of magnesium to oxygen
 This picture representes Mole ratio of magnesium to oxygen.
This picture representes Mole ratio of magnesium to oxygen.
Magnesium oxide experiment errors
 This image representes Magnesium oxide experiment errors.
This image representes Magnesium oxide experiment errors.
Empirical formula magnesium oxide experiment
 This image shows Empirical formula magnesium oxide experiment.
This image shows Empirical formula magnesium oxide experiment.
Empirical formula magnesium oxide lab
 This picture shows Empirical formula magnesium oxide lab.
This picture shows Empirical formula magnesium oxide lab.
How to determine the formula for magnesium oxide?
The objective of this lab is to experimentally determine the empirical formula of Magnesium Oxide. The objective of this lab is to experimentally determine the empirical formula of Magnesium Oxide. 1. Setup ring stand with five inch ring and triangle. 2. Obtain desired amount of Magnesium, a crucible with a lid and crucible tongs.
What was the objective of the magnesium oxide experiment?
One objective was to figure out if the burnt Mg ashes weigh more than the product which is Magnesium Metal. Another objective was determining the formula of the compound that results when Magnesium and Oxygen react. Theory:
How old do you have to be to do magnesium oxide lab?
The amount of MgO formed will be dependent on the amount of Magnesium ribbon used, along with avoidance of human error (i.e incorrect weighing or loss of experimental product). This lab can be performed as an individual or with a partner (Recommended for Grade 10 and above)
What is the result of combining magnesium with oxygen?
This experiment involves combining Magnesium with Oxygen which is a gas represented by the chemical element 8. The word equation for the result of this experiment is Magnesium + Oxygen= Magnesium Oxide. The equation is Mg + O =MgO Definitions Word Definitions Mass Number The Mass number is the amount of Protons and Neutrons in the Nucleus.
Last Update: Oct 2021